Database Analysis of Weak Interactions in Protein Architecture
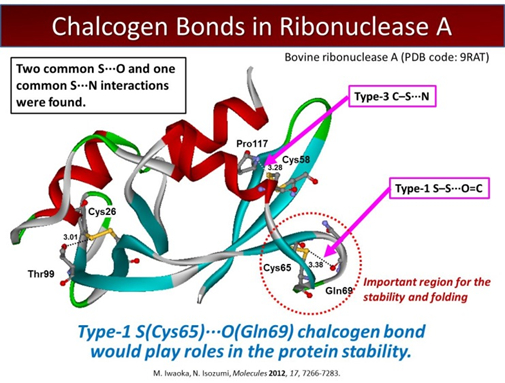
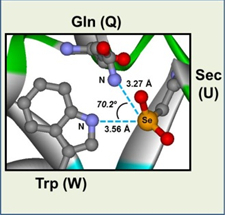
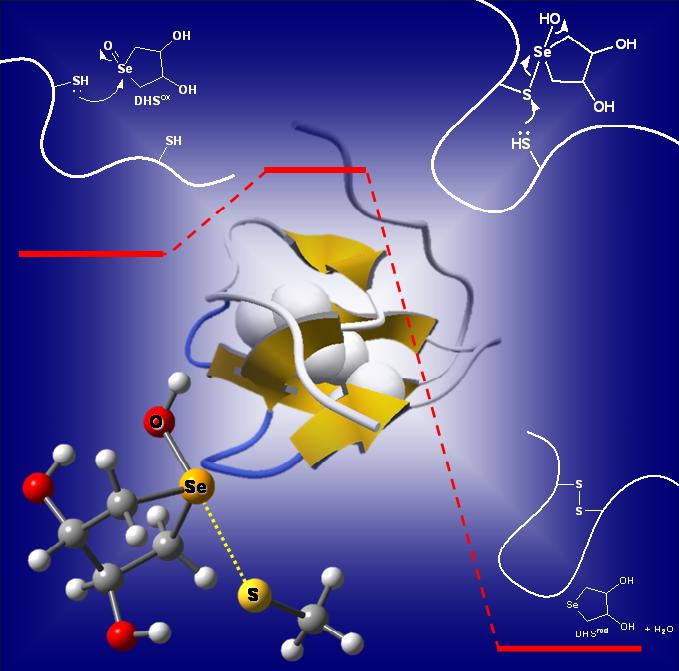
Nonbonded interactions between a divalent sulfur atom and polar functional groups, i.e., S···X (X = O, N, and S) interactions, have recently been demonstrated to stabilize protein structures to some extent and play putative roles in their function and evolution. The directional features observed between the interacting S and X atoms suggest that they can also be called S···X chalcogen bonds in analogy to halogen bonds. We are interested in characterization and energetic evaluation of such weak atomic interactions, which should play important roles in folding and stabilization of the three-dimensional protein architecture.