Selenosugar
A New Series of Selenosugar Molecules has been Synthesized (June, 2021)
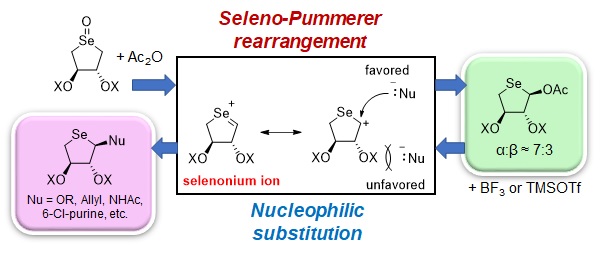
Selenosugars, carbohydrate compounds having a selenium atom, are interesting targets of drug discovery because they are expected to possess unique biological activities arising from the introduced selenium atom. Many kinds of selenosugars and selenonucleosides as well have already been synthesized, but selenothreose, which has two substituents at the 2- and 3-positions of a five-membered 4-selenofuranose ring in a trans configuration, has not been reported in the literature. In our group, we studied the synthesis of a new series of 4-selenothreofuranose derivatives using DHS as a starting material. DHS is a water-soluble 5-membered cyclic selenide that is developed in our laboratory. After oxidation of DHS, Seleno-Pummerer rearrangement was applied to the obtained selenoxide. The target compounds were yielded in high yields. The obtained selenosugars were then converted to various 4-selenothreoglycosides by the reaction with a nucleophile in the presence of a Lewis acid. We further demonstrated the synthesis of 4'-selenothreonucleoside derivatives using a nucleobase (6-chloropurine) as a nucleophile. The achievements were published in ACS Omega, an open access journal of the American Chemical Society. For details, click the following link, http://doi.org/10.1021/acsomega.1c02160