Recent Activity
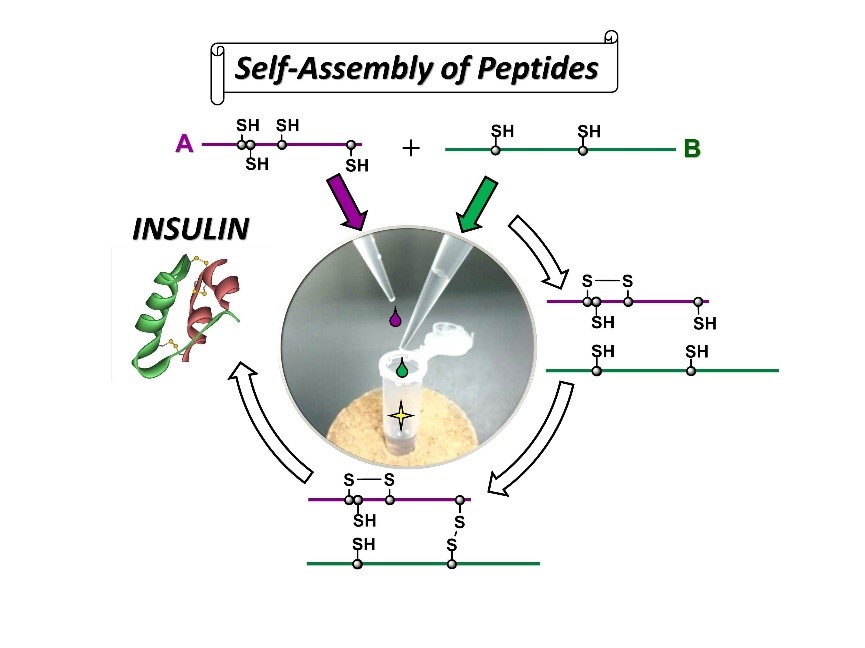
Two-chain folding pathways of insulin has been elucidated clearly (May, 2018)
Chemical synthesis of insulin has been a long challenge in scientific research because insulin, an important pancreatic hormone regulating a postprandial glucose level in blood, has characteristic structure composed of two peptide chains (i.e., A-chain and B-chain) that are connected by two disulfide (SS) crosslinks. Moreover, there is another SS bond in the A-chain. Therefore, when the two peptide chains are mixed in solution, a number of SS-bonded species are produced. Now, we have characterized SS bonds present in each species and thus succeeded in elucidation of the two-chain folding pathways of insulin unambiguously. By optimizing the two-chain assembly conditions based on the revealed folding pathways, native insulin can be obtained in up to 49% yield just by mixing the A- and B-chains without application of any protective groups in the peptide chains. The achievements have been published in Communications Chemistry. To access the paper, click here.