Recent Activity
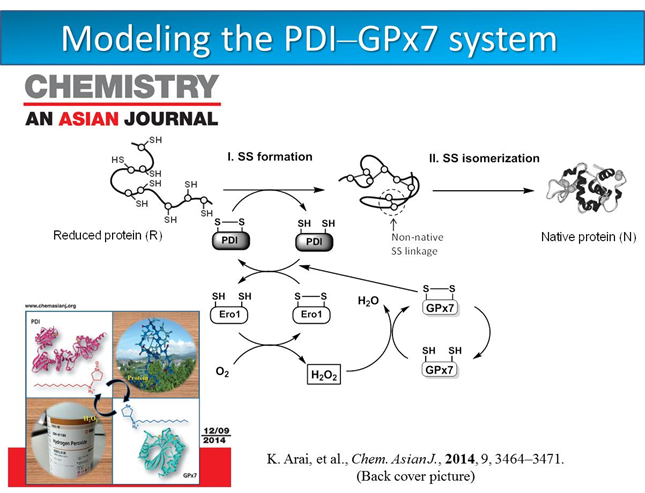
Dr. Kenta Arai’s paper was highlighted as a back cover picture in Chemistry an Asian Journal. (November, 2014)new!
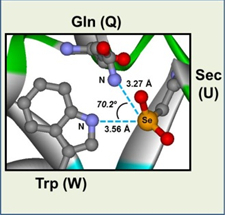
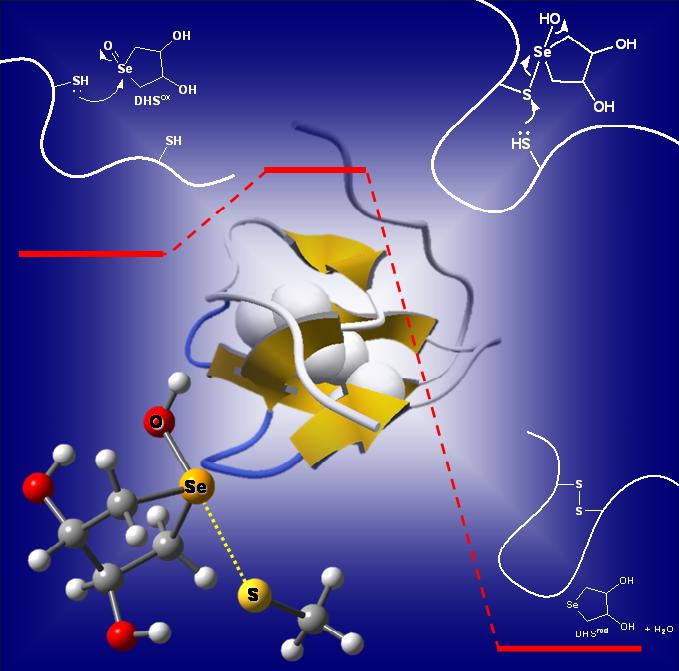
A nascent protein must be folded into a specific three-dimensional structure to exhibit the biological activity. In this protein-folding process, not only the conformational conversion of the polypeptide chain but also the disulfide (SS) formation between the two cysteine residues is generally required. We have designed amphiphilic selenides, which have significant affinity toward the hydrophobic regions of a reduced protein and can effectively catalyze SS formation in the presence of H2O2, eventually leading to the native fold. The functions of the designed amphiphilic selenides are similar to those exhibited by PDI and GPx7, which enhance the folding efficiency of nascent proteins and maintain ROS homeostasis, respectively, in eukaryotic ER. The combination of a water-soluble selenide and long-chain alkyl group can be a useful motif for designing medicines for both protein-misfolding diseases and antioxidant therapy.